Engineering units
After taking the engineering units you will be able to answer questions such as:
- Why do Indians put their ear to the tracks to hear if the train is coming?
- What is milk?
- How can a mosquito walk on water and not sink?
- When we go to a fair, why we can buy a balloon in a different shape - a dog, a rabbit, or a donut?
- When to boil the rakia (Bulgarian fruit brandy)?
- Why do Formula One cars have wings but do not fly?
Following these units, you will acquire the skills needed to control and optimize chemical processes. You will learn the chemical and physical phenomena that occur both in the beaker and in the production reactors. Some of these include the laws of heat transfer (energy), how bodies resist flow (rheology), the formation and breakdown of disperse systems (mayonnaise, ice cream, shaving cream), and the control of chemical processes.
In the industry, work is done on a much larger scale than in the laboratory. Therefore, it is necessary to optimize the processes using the aforementioned theoretical knowledge. As the goal is to reduce the production time, the productivity is optimal, and the costs are minimal.
Units from this Section
Transfer Phenomena
The Transport Phenomena unit teaches the mechanisms and the mathematical equations that describe the energy transfer processes such as the transport of mass (diffusion), or of heat (convection, conduction, radiation).
In the first part of the unit, students get the basic theoretical and experimental knowledge of heat transfer in a stationary medium, and in a convective flow; free convection; the effect of heat sources (viscous, electric; chemical, nuclear); finding the temperature distribution in different modes of heat exchange.
In the second part of the unit, students gain not only basic theoretical and experimental knowledge of diffusion and convection in multicomponent systems, but also more in-depth knowledge of diffusion processes in different external fields, in homogeneous and heterogeneous chemical reactions, in adsorption and interphase mass transfer.
Continuum Mechanics and Rheology

This unit studies the behavior of fluids when subjected to different force fields (gravity, pressure, etc.). Rheology is defined as the science of deformation and flow of materials. The main topics in the unit are as follows: mass balance, momentum and kinetic energy; strain and shear rate; laminar and turbulent flows; and the difference between Newtonian and non-Newtonian fluids.
At the seminar classes, students will acquire the necessary skills to solve the problems discussed during the lectures. During the lab practice students obtain laboratory skills while working with the most used rheological instruments in the industry. Some of the simple types of fluid flows are studied in practice.
You will learn why, when it rains, sound reaches us faster, why when you hit the surface of the water hard, it feels like a solid body, and many other intriguing phenomena.
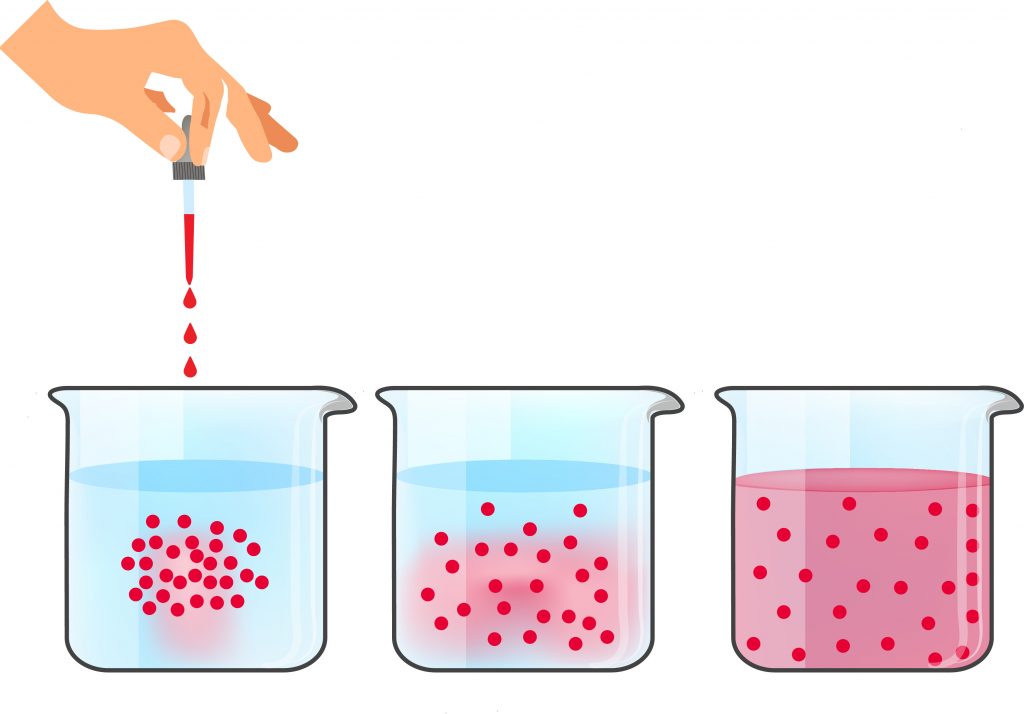
Chemical kinetics
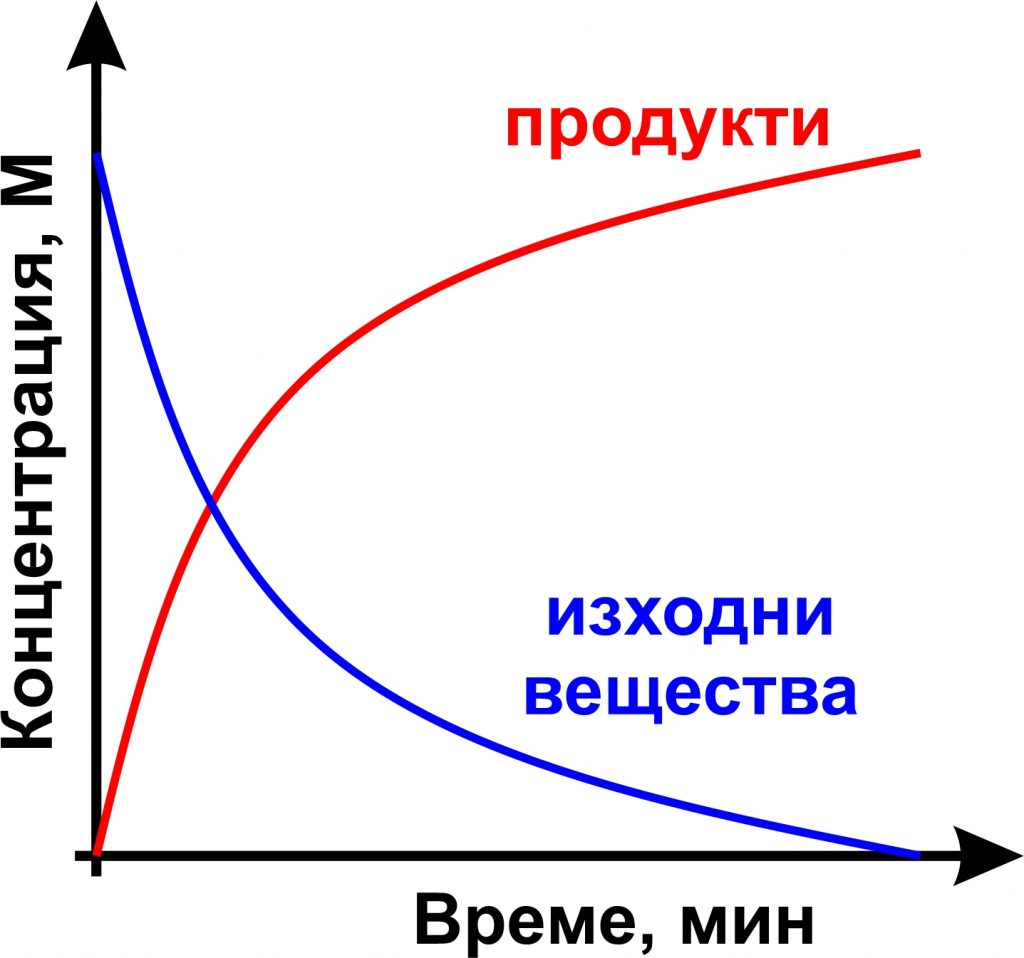
The unit aims to provide students with in-depth knowledge of the basic concepts and approaches in chemical kinetics, as well as basic knowledge of the application of kinetic laws in the description of chemical reactors. Topics: reactions in solutions; adsorption and reactions on a surface; homogeneous and heterogeneous catalysis; influence of mass and heat transfer on the rate of chemical reactions; kinetics in chemical reactors. In all sections, specific examples are given to illustrate the basic patterns and their applications, and relevant experimental methods are discussed.
Disperse systems
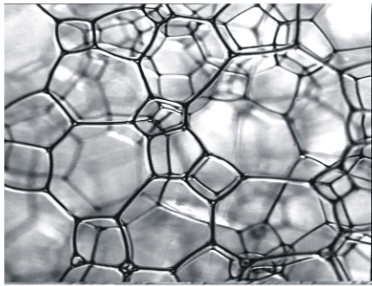
The aim of the unit is to acquaint students with the main types of disperse systems that are used in industry and household products. Students acquire theoretical knowledge and practical skills related to the preparation of foams, emulsions, and suspensions, and methods for studying their structure and stability. During the lectures in the unit, you will study the main physicochemical aspects and applications of surface-active substances, the role of surface forces for the stability of disperse systems, and electrokinetic phenomena.
Students learn about the main processes leading to the destruction of dispersions (sedimentation, coagulation, coalescence, syneresis, etc.) and the factors determining the speed of these processes.
Computer modeling
In this unit, you will acquire the basic knowledge you need to create mathematical models that describe real processes. You will be able to create computer algorithms that can be used to make accurate calculations based on experimental results and predict future ones.

Separation processes

The aim of this unit is for students to learn about the types of disperse systems, the factors that determine their properties, and about the separation processes. The stability issues of different systems, the role of the interfacial tension, adsorption, and surface forces, and the factors that lead to coagulation, flocculation, or Ostwald ripening are considered.
Special attention is paid to the membrane separation processes, depth filtration, gravity, and inertial sedimentation, as well as the separation methods for aerosols. In addition, the unit introduces students to basic concepts such as separation efficiency, particle size distributions, coagulation kinetics, the use of surfactants to modify the surface properties of dispersed particles, etc. The lab practice illustrates the lecture material and gives a visual idea of the studied systems and processes.
