Introductory units
In the first year of the Chemical Engineering and Contemporary Materials program students attend lectures in a series of introductory units such as general chemistry, physics, mathematics, English, and statistics. The purpose of these units is to build the foundation necessary to understand the material from the fundamental and specialized units studied in the following years.
Units from this Section
Units from this Section
General Chemistry

In this unit, students get to know the different aspects of chemistry, including structure of the atom, the periodic table of chemical elements, the basic laws of thermodynamics, chemical kinetics and many more. The acquired knowledge is deepened during the next semesters of study – the knowledge from (almost) each individual lecture is expanded through an independent unit.
"By studying quantum mechanics, one can become convinced that this is a world completely different from the one we observe with the naked eye – there, the known laws of mechanics do not apply. An example of this is the famous paradox of Schrödinger's cat: a cat is placed in box along with a radioactive element, and the lid of the box is closed. After a certain time, the cat is just as likely to be alive as it is not. To a bystander, the cat is both alive and dead at the same time. The case can only be solved if one opens the box and looks inside. This is how things are in the quantum world - the position of very small particles (the electron, for example) cannot be clearly defined - it can be both 'here' and 'there'. Its position can only be described by some probability function—that's the electron orbital."
General physics

This unit introduces students to classical physics and the basic principles of mechanics (velocity, acceleration, force, energy), mechanical waves (e.g. sound), thermodynamics, electricity and magnetism, as well as light and its characteristics. At the end of the unit, the theory of relativity is considered. These basic principles are at the basis of the description of all specific processes and phenomena studied in the further course of study.
Informatics, computers and statistics
This unit studies the techniques needed to work effectively with experimental data and their statistical interpretation. It is especially important in practical work, since the chemist's work often involves conducting experiments - either in the laboratory, or theoretically on a computer. The resulting experiments usually represent large sets of data, in which the values of a given quantity are depending on another (for example, the outside temperature depends on the time when it is measured), which need to be interpreted and presented graphically.
Mathematics
The purpose of this unit is to build on the mathematical knowledge acquired during high school. Complex numbers, matrices, limits, functions of one and several variables, derivatives, integrals, differential equations, and others are considered. Mastering these mathematical techniques is particularly important for further study, as they are the main way to describe the processes and phenomena studied in the fundamental and specialized units.
"Most often, the rate of a chemical reaction is determined by an experiment in which the change in the concentration of substances with time is measured, i.e., their derivative. To be able to determine how the concentration changes with time after a few days (without you needing to spend several days in the laboratory), you need to derive a functional dependence of the concentration with time. This is possible by solving a differential equation."
English
The English language unit aims to familiarize students with the basic terms used in scientific literature in the field of chemistry and physics.
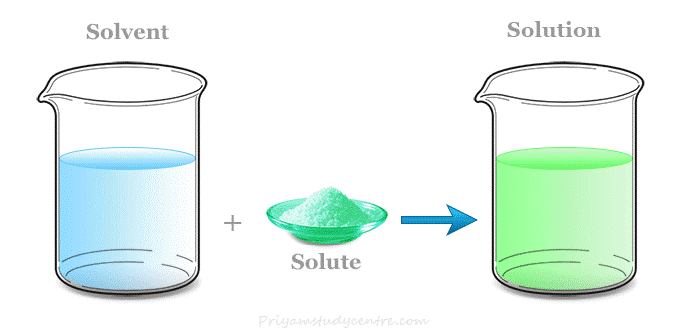
Two types of “solutions”